BioCardia Announces Positive DSMB Review and Recommendation to Continue Phase III Pivotal CardiAMP Cell Therapy Heart Failure Trial

SUNNYVALE, Calif., Aug. 31, 2022 (GLOBE NEWSWIRE) — BioCardia®, Inc. [Nasdaq: BCDA], a developer of cellular and cell-derived therapeutics for the treatment of cardiovascular and pulmonary disease, today announces that the independent Data Safety Monitoring Board (DSMB) has completed its prespecified data review for the ongoing Phase III pivotal CardiAMP® Cell Therapy Heart Failure Trial (clinicaltrials.gov Identifier: […]
Stem cells saving lives from heart birth defects
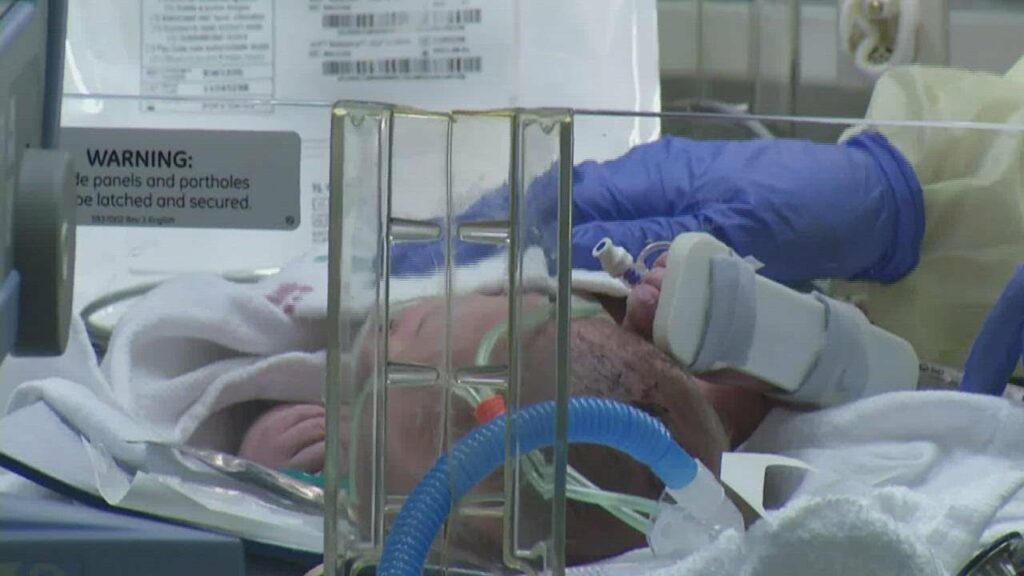
Stems Cells are being used to help revitalize the hearts of babies who suffer from a severe heart defect and sometimes prevent the need for a transplant. Video Transcript: Almost 1 out of 100 babies is born with a heart defect. Each year in the U.S. many of these babies will need surgery within weeks […]
Creative Medical Technology Announces Progress in Developing a Reproducible Clinical Grade of the Company’s ImmCelz® Product

PHOENIX, April 20, 2022 /PRNewswire/ — Creative Medical Technology Holdings, Inc. (“Creative Medical Technology” or the “Company”) (NASDAQ: CELZ), a leading commercial stage biotechnology company focused on a regenerative approach to immunotherapy, urology, neurology, and orthopedics, today announced progress in developing a reproducible clinical grade product of ImmCelz®. Multiple clinical experiments were performed in which […]