Will CPI Stem Cells Achieve FDA Approval? Scotty Nelson Discusses the Future of Regenerative Medicine

In a recent discussion, Scotty Nelson, co-founder of the Cellular Performance Institute (CPI) Stem Cells, delved into the complexities and potential of stem cell therapy, particularly focusing on the possibility of FDA approval for CPI’s innovative treatments. CPI Stem Cells, located in Tijuana, Mexico, is at the forefront of regenerative medicine, offering advanced stem cell […]
Revolutionizing ALS Treatment: BrainStorm’s Phase 3b Trial Moves Forward with FDA Approval

BrainStorm Cell Therapeutics Inc., a leader in adult stem cell therapeutics for neurodegenerative diseases, has achieved a significant milestone with the FDA’s agreement on a Special Protocol Assessment (SPA) for its Phase 3b trial of NurOwn® in amyotrophic lateral sclerosis (ALS). This agreement marks a critical step in advancing the potential treatment to the market, […]
NFL Star Kirk Cousins’ Revolutionary Recovery: Embracing Stem Cell Treatment in Antigua for Achilles Healing
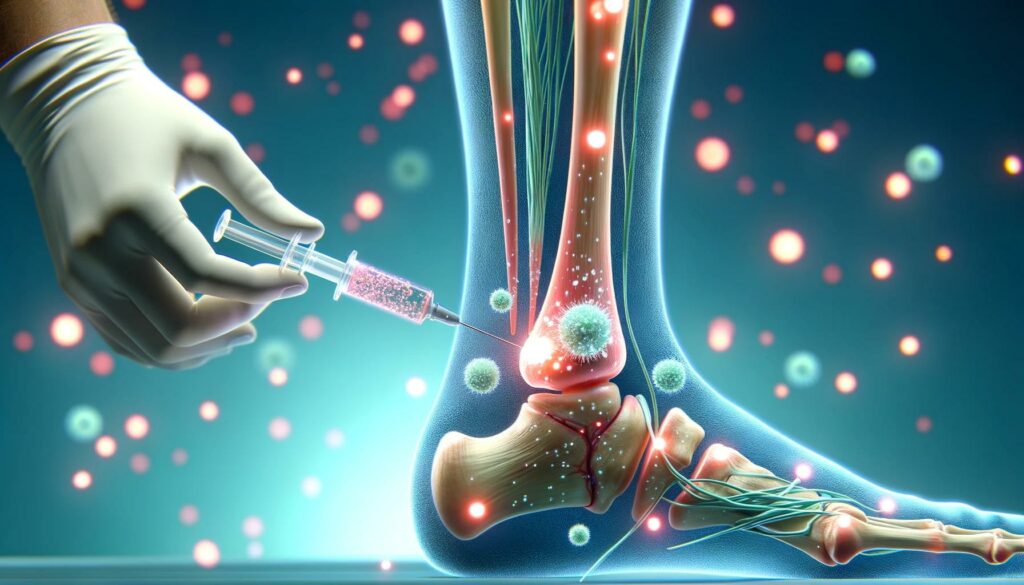
Kirk Cousins, the quarterback for the Minnesota Vikings, recently traveled to Antigua for stem cell treatment following a devastating Achilles injury he suffered in October. This journey highlights the growing interest and use of stem cell therapy in sports medicine, particularly for recovery and enhancement of overall health. The video from KARE 11 News details […]