In a groundbreaking study, NeuroSense’s PrimeC has demonstrated a significant effect on the survival rate of induced motor neurons in an innovative in vitro model.
This research was conducted in collaboration with the University of Southern California’s Ichida Stem Cell Lab. The study utilized induced pluripotent stem cells (iPSCs) derived from individuals diagnosed with ALS.
Key Highlights:
- PrimeC was found to substantially enhance the survival rate of induced motor neurons in an in vitro study using iPSCs from ALS patients.
- This recent study corroborates earlier findings from multiple models.
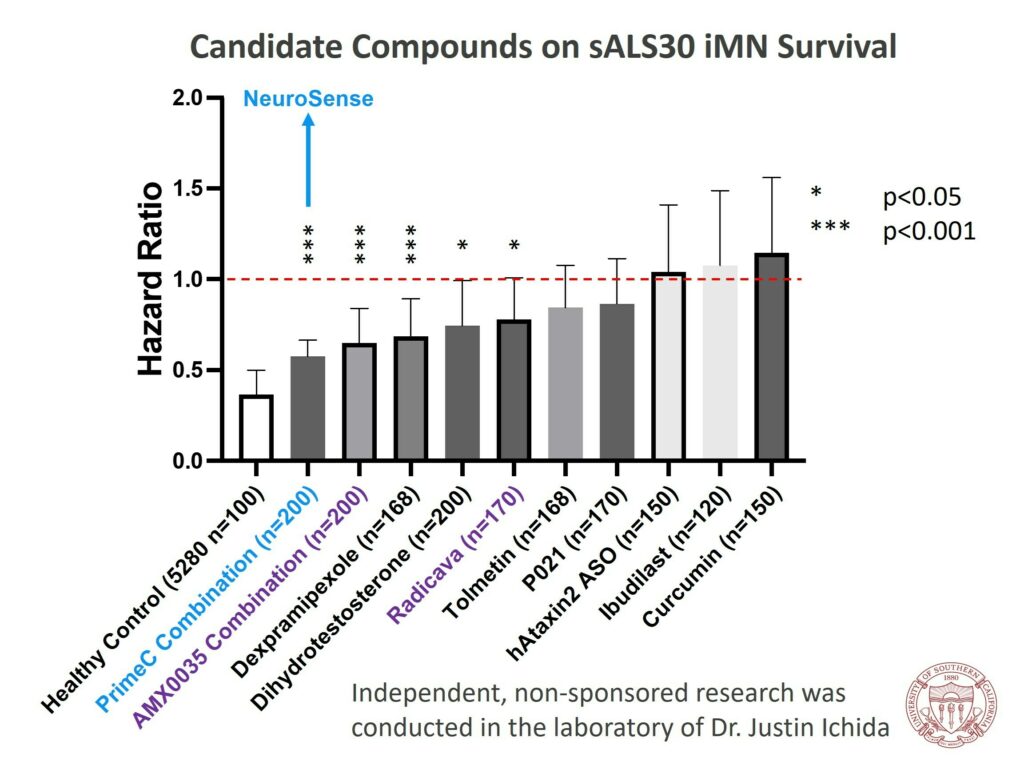
- In a separate study by Dr. Ichida, PrimeC was among the top performers in enhancing motor neuron survival, outperforming several other ALS drugs under development and two FDA-approved ALS medications.
- NeuroSense Therapeutics Ltd., a firm dedicated to developing treatments for severe neurodegenerative diseases, announced these findings.
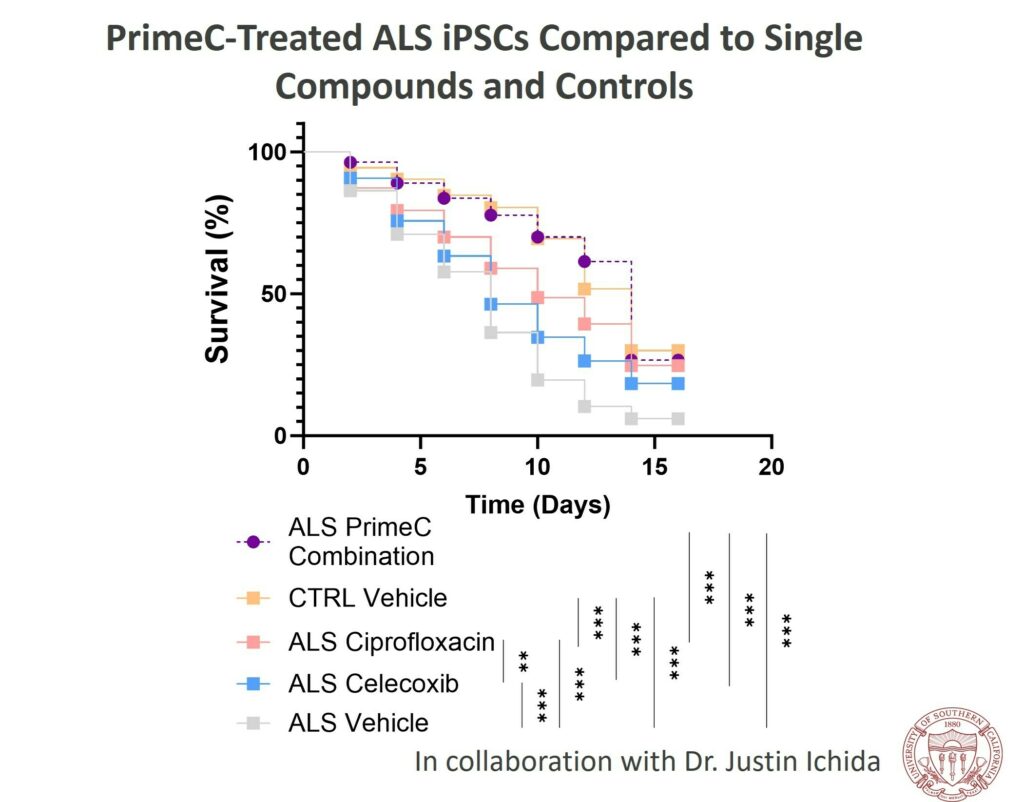
- The study revealed that PrimeC surpassed ciprofloxacin and celecoxib, aligning the neuronal survival rate with that of healthy controls.
- Dr. Ichida’s study employed iPSCs derived from ALS patients’ blood samples. The research evaluated the survival rate of induced motor neurons treated with PrimeC compared to its individual components, ciprofloxacin and celecoxib. The results highlighted PrimeC’s synergistic effect, emphasizing the rationale behind combining the two active ingredients in NeuroSense’s proprietary formulation.
- An earlier study by Dr. Ichida had already shown that NeuroSense’s combination therapy was among the best in terms of enhancing motor neuron survival, alongside other ALS drugs in development and two FDA-approved ALS drugs.
Dr. Ichida expressed his excitement about the results, stating that the data from the iPSC in vitro study of PrimeC was impressive. He further mentioned that PrimeC’s combination was among the best in enhancing motor neuron survival, and its synergistic effect exceeded expectations.
NeuroSense’s Founder and CEO, Alon Ben Noon, expressed gratitude to Dr. Ichida and his team for evaluating PrimeC in this non-sponsored study. He emphasized the company’s confidence in PrimeC as a potential therapy for ALS.
Dr. Shiran Zimri, VP R&D of NeuroSense, highlighted the potential of using iPSCs as a non-invasive method to screen patients who are most likely to benefit from PrimeC.
NeuroSense anticipates revealing clinical topline results from its Phase 2b study of PrimeC in ALS treatment by the end of 2023.
About NeuroSense: NeuroSense Therapeutics Ltd. is a clinical-stage biotech firm focused on discovering and developing treatments for patients suffering from debilitating neurodegenerative diseases, including ALS, Alzheimer’s disease, and Parkinson’s disease.
Source: NeuroSense