Revolutionizing Crohn’s Disease Treatment: TRX103 Gains FDA Clearance
Tr1X, a trailblazing biopharmaceutical company, has announced a significant milestone in the fight against treatment-refractory Crohn’s Disease. The FDA has granted clearance for the Investigational New Drug (IND) application of their innovative therapy, TRX103, an allogeneic engineered Tr1 Treg cell therapy. This breakthrough offers new hope for patients who have not responded to conventional treatments. […]
Longeveron Unveils Promising Phase 2a Results for Regenerative Medicine and Cell Therapy in Mild Alzheimer’s Disease at AAIC 2024

Longeveron Inc., a leader in regenerative medicine and cell therapy, recently presented groundbreaking findings from their Phase 2a CLEAR MIND trial of Lomecel-B™ at the Alzheimer’s Association International Conference (AAIC) 2024. The trial focused on the efficacy and safety of Lomecel-B™, a medicinal signaling cell therapy, in treating mild Alzheimer’s Disease. Key Highlights: Implications for […]
FDA Accepts BLA for Ryoncil: First Allogeneic “Off-the-Shelf” Cellular Medicine and First Cell Therapy for Children in the United States

In a significant milestone for pediatric medicine, the FDA has accepted Mesoblast’s Biologics License Application (BLA) for Ryoncil® (remestemcel-L), aimed at treating children with Steroid-Refractory Acute Graft-Versus-Host Disease (SR-aGVHD). This acceptance marks a crucial step towards the approval of the first allogeneic “off-the-shelf” cellular medicine in the United States, and the first cell therapy for […]
Breakthrough in Muscular Dystrophy Treatment: FDA Approves Stem Cell Therapy from Myogenica
The fight against muscular dystrophy has taken a promising turn with the recent FDA approval of an Investigational New Drug (IND) application for MyoPAXon, a revolutionary stem cell-derived muscle stem cell product developed by Myogenica, a University of Minnesota startup. This landmark approval paves the way for a clinical trial aimed at treating patients with […]
Breakthrough in Alzheimer’s Treatment: Lomecel-B™ Receives RMAT Designation from FDA

Longeveron Inc., a clinical stage regenerative medicine biotechnology company, has achieved a significant milestone in the fight against Alzheimer’s Disease. The U.S. Food and Drug Administration (FDA) has granted Regenerative Medicine Advanced Therapy (RMAT) designation to Lomecel-B™ for the treatment of mild Alzheimer’s Disease. This designation underscores the potential of Lomecel-B™ to provide a new […]
Groundbreaking Progress in GvHD Treatment: Tr1X Successfully Doses First Patient in TRX103 Trial

In an exciting development for autoimmune and inflammatory disorders, Tr1X, Inc. has announced the successful dosing of the first patient in its TRX103-01 proof-of-concept trial. This trial is a significant step forward in the fight against Graft versus Host Disease (GvHD), a serious condition affecting patients undergoing mismatched hematopoietic stem cell transplantation (HSCT). TRX103 represents […]
KOSÉ, I Peace, and Reju to Revolutionize Personalized Beauty with iPS Cells
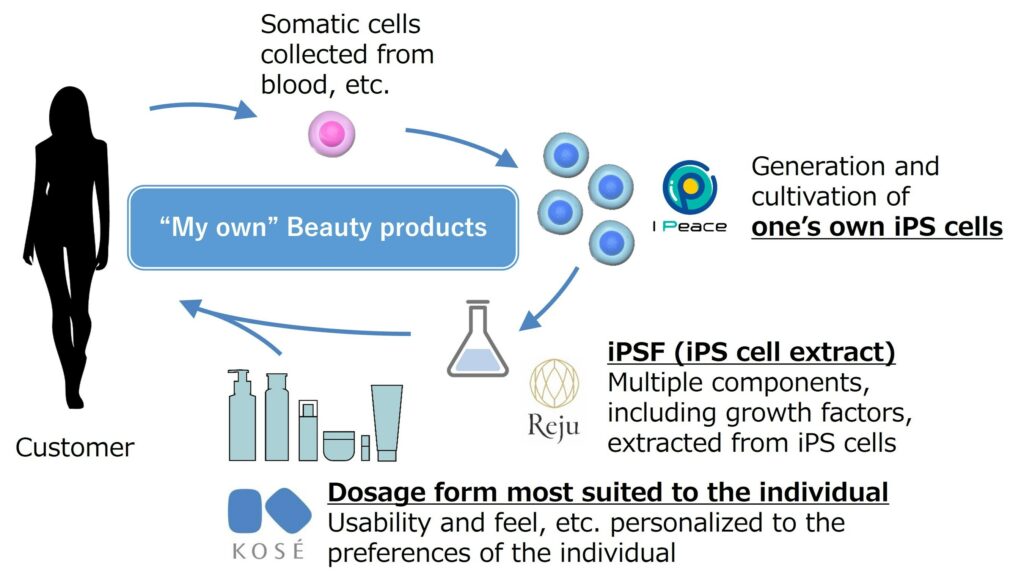
In a groundbreaking move, KOSÉ Corporation, I Peace, Inc., and Reju, Inc. have announced a strategic partnership aimed at developing personalized beauty products using iPS cells. This collaboration brings together the expertise of KOSÉ in beauty products, I Peace’s advancements in iPS cell technology, and Reju’s innovation in iPSF (iPS cell extract) to create customized […]
Senti Bio Receives $8 Million CIRM Grant to Advance Logic-Gated CAR-NK Cell Therapy for AML

Senti Biosciences, Inc., a leader in next-generation cell and gene therapies, has been awarded an $8 million grant by the California Institute for Regenerative Medicine (CIRM). This significant funding will propel the clinical development of SENTI-202, an innovative off-the-shelf CAR-NK cell therapy designed to treat relapsed/refractory hematologic malignancies, including acute myeloid leukemia (AML). Pioneering Cell […]
Revolutionary Stem Cell Treatment for Hair Loss: Eirion Therapeutics Leads the Way

Eirion Therapeutics, a pioneer in stem cell research, has announced the commencement of a groundbreaking Phase 1 clinical trial to evaluate the safety of ET-02, a topical pharmaceutical designed to treat androgenic alopecia, commonly known as age-related hair loss. This double-blind, placebo-controlled study marks a significant milestone in the treatment of hair loss, enrolling the […]
BrainStorm Cell Therapeutics Aligns with FDA on Phase 3b NurOwn Clinical Trial CMC Aspects

BrainStorm Cell Therapeutics Inc., a leading developer of adult stem cell therapeutics for neurodegenerative diseases, has reached an important milestone by aligning with the U.S. Food and Drug Administration (FDA) on the Chemistry, Manufacturing, and Controls (CMC) aspects of its Phase 3b clinical trial for NurOwn®, an investigational therapy for amyotrophic lateral sclerosis (ALS). This […]