Age-Reversal for Stem Cells: What Mount Sinai’s Lysosome Breakthrough Means

A New Hope for Healthy Aging Scientists at the Icahn School of Medicine at Mount Sinai have made a groundbreaking discovery, reversing the aging process in blood-forming stem cells in mice. This breakthrough, published in Cell Stem Cell, offers new hope for treating age-related blood disorders and potentially even slowing the overall aging process. The key […]
Cellipont and Ronawk Forge Alliance to Revolutionize MSC Expansion for Regenerative Medicine

A landmark strategic collaboration has been announced between CelliPont Bioservices, a leading cell therapy Contract Development and Manufacturing Organization (CDMO), and Ronawk, the innovative developer of the Bio-OS™ biological operating system. This partnership, unveiled on November 19, 2025, is set to tackle some of the most significant hurdles in regenerative medicine by accelerating the scalable, […]
Maryland Announces FY26 Stem Cell Research Funding

Maryland Backs Innovation in Regenerative Medicine The Maryland Stem Cell Research Commission (MSCRC) has announced its second funding cycle for fiscal year 2026 (FY26). [1] This initiative invites researchers and companies to submit proposals that will accelerate stem cell research, its translation into therapies, and commercialization within Maryland. [1] This funding is a key part […]
Pluristyx Announces Two Key Licensing Agreements for iPSC Portfolio

Pluristyx, a biotechnology company, has executed two commercial clinical use licensing agreements. These agreements provide access to Pluristyx’s clinical-grade iPSC lines and gene engineering platform technologies. The goal is to develop next-generation cell therapies. Advancing Neurological and Regenerative Therapies One agreement is with a company developing a treatment for a neurological disease. This partnership will […]
IN8bio Expands Leukemia Clinical Trial, Adds Ohio State University as New Site
IN8bio, a clinical-stage biopharmaceutical company, has expanded its Phase 1 clinical trial for INB-100. The Ohio State University is now a new clinical site for the trial. This expansion aims to accelerate enrollment and completion of the study. INB-100 Clinical Trial The INB-100 trial is evaluating a donor-derived gamma-delta T cell therapy for leukemia patients […]
The Jackson Laboratory Acquires New York Stem Cell Foundation to Revolutionize Biomedical Research
The Jackson Laboratory (JAX), a global leader in genetics and genomic medicine, has acquired the New York Stem Cell Foundation (NYSCF). This strategic unification creates a powerful nonprofit engine for biomedical discovery, poised to accelerate the development of precision therapies for a wide range of diseases. Key Details of the Acquisition The acquisition, finalized on […]
A Tiny Worm’s Regenerative Abilities Could Reshape Human Healing
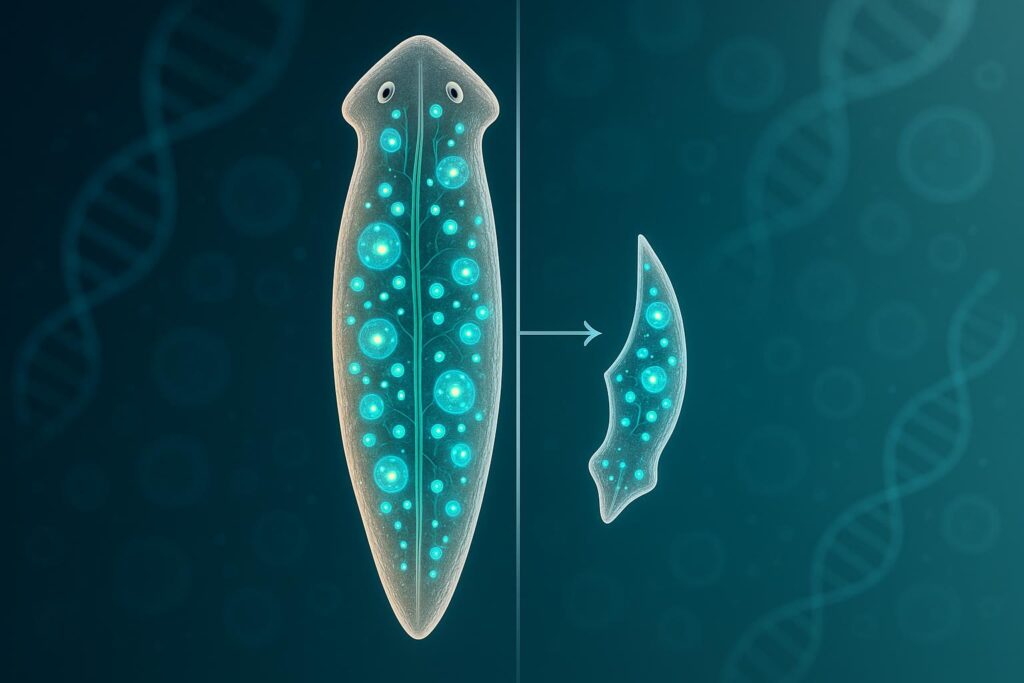
Unlocking the Secrets of Stem Cells Have you ever wondered if the human body could heal itself more effectively? New research on a tiny worm offers surprising insights into this question. The study focuses on the planarian flatworm, an animal with an extraordinary ability to regenerate. This research challenges long-held beliefs about how stem cells […]
Pluristyx and BioLamina Forge Strategic Alliance to Propel Stem Cell Therapies Forward

A new partnership between Pluristyx and BioLamina promises to accelerate the development of stem cell-based therapies. This collaboration will simplify and de-risk the path to clinical translation for these innovative treatments. In a significant move for the regenerative medicine field, Seattle-based Pluristyx Inc. and Swedish company BioLamina AB have announced a strategic co-development and co-marketing […]
Free, On-Demand Course on Human Stem Cell Research Standards (ISSCR × STEMCELL Technologies)

Clear standards make better science. That’s why the International Society for Stem Cell Research (ISSCR) has partnered with STEMCELL Technologies to launch a free, on-demand course that shows researchers how to apply the ISSCR Standards for Human Stem Cell Use in Research. The course is slated for fall 2025 and is designed for scientists, students, […]
SB 1768 Opens Door to Non-FDA-Approved Stem Cell Therapies: What Patients Need to Know

Are you wondering what SB 1768 means for your access to stem cell treatments? Have you heard about new state laws allowing non-FDA-approved therapies? Do you want to understand how these changes might affect your treatment options for orthopedic conditions, wound care, or chronic pain? These questions reflect growing interest in recent state legislation that […]