Brain Cell Implants: A New Hope for Parkinson’s Patients?
A groundbreaking clinical trial is testing whether stem cell implants can restore brain function in people with Parkinson’s disease, offering a potential new path to slow the disease and improve quality of life. For the more than one million people in the United States living with Parkinson’s disease, treatment options have been limited to managing […]
Stem Cell Therapy for Parkinson’s Disease: Breakthrough Clinical Trials Offer New Hope

Will stem cell therapy finally offer a cure for Parkinson’s disease? Can lost brain cells truly be replaced? These are the questions that millions of patients and families ask as they search for better treatment options. Recent clinical trials have brought us closer to answering these questions. Leading experts Dr. Jun Takahashi and Dr. Claire […]
Aspen Neuroscience’s Personalized Parkinson’s Therapy Shows Promise in Clinical Trial

For the millions of people living with Parkinson’s disease, the search for effective treatments is a constant source of hope and concern. Current therapies manage symptoms but do not stop the progression of the disease. However, recent advancements in cell therapy are offering a new frontier in the fight against Parkinson’s. A New Era for […]
Kenai Therapeutics Gets $8M Boost for Parkinson’s Treatment
A New Hope for Parkinson’s Patients Kenai Therapeutics, a San Diego-based biotechnology company, has received an $8 million grant from the California Institute for Regenerative Medicine (CIRM). This funding will help advance their lead program, RNDP-001, a promising new treatment for idiopathic Parkinson’s disease. The grant was awarded to Dr. Howard J. Federoff, the company’s […]
Parkinson’s Symptoms Reversed: A Stem Cell Breakthrough?

For the 10 million people worldwide living with Parkinson’s disease, the daily battle with tremors, stiffness, and loss of motor control is a constant reality. But a groundbreaking clinical trial in China has delivered a powerful new sense of hope, as a 37-year-old woman has achieved a “functional cure” just three months after receiving a […]
New Hope for Parkinson’s Patients: Cell Therapy Shows Promise
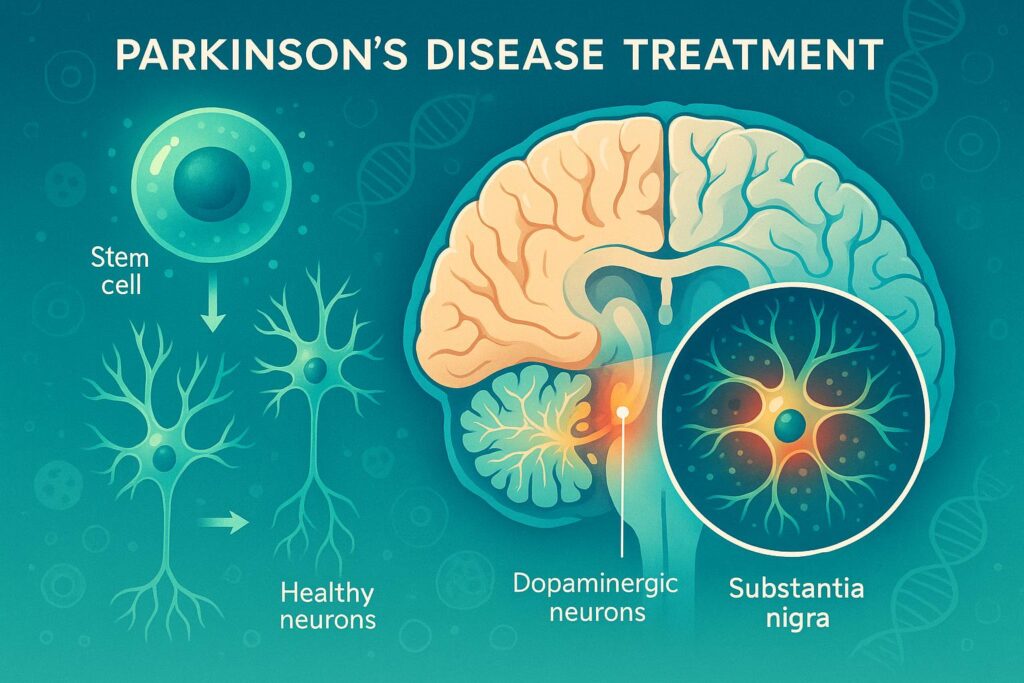
For individuals with Parkinson’s disease (PD), the progressive loss of motor function presents a daily challenge. The search for effective treatments has led researchers to explore cell replacement therapy, and recent findings have generated significant optimism. New Studies Report Positive Trends Two studies, released at the International Congress of Parkinson’s Disease and Movement Disorders®, have […]
Aspen Neuroscience Advances Parkinson’s Treatment with New Trial Phase
More than one million people in the U.S. live with Parkinson’s disease, a condition with no available disease-modifying therapies. A new development from Aspen Neuroscience offers a potential new direction, as the company initiates a new phase in its clinical trial for a personalized cell therapy. ASPIRO Trial Enters Third Cohort Aspen Neuroscience has announced […]
BlueRock Therapeutics Announces First Patient Treated in Pivotal Phase III Parkinson’s Trial
BlueRock Therapeutics, a subsidiary of Bayer, has announced a significant milestone in the development of a new treatment for Parkinson’s disease. The first patient has been treated in their pivotal Phase III clinical trial, known as exPDite-2. This trial will evaluate the safety and effectiveness of bemdaneprocel, an investigational cell therapy. The trial is the first […]
Stem Cell Research for Parkinson’s: What Patients Need to Know

Is stem cell therapy a viable treatment for Parkinson’s disease? What are the real risks and benefits? In a recent webinar from The Michael J. Fox Foundation for Parkinson’s Research, leading experts shared the latest advances in stem cell science and what they mean for patients. This article summarizes the key takeaways from the webinar, […]
Trailhead Biosystems Launches New iPSC-Derived A9 Dopaminergic Neurons to Advance Parkinson’s Research

Trailhead Biosystems, Inc., a biotechnology company focused on creating induced pluripotent stem cell (iPSC)-derived human cells for drug discovery and cell therapy, today announced the launch of TrailBio® A9 Dopaminergic Neurons. This new product provides researchers with a critical tool for studying Parkinson’s disease and other neurodegenerative conditions. A9 dopaminergic neurons are essential for regulating […]